Riverside offers vital option for women with dense breasts
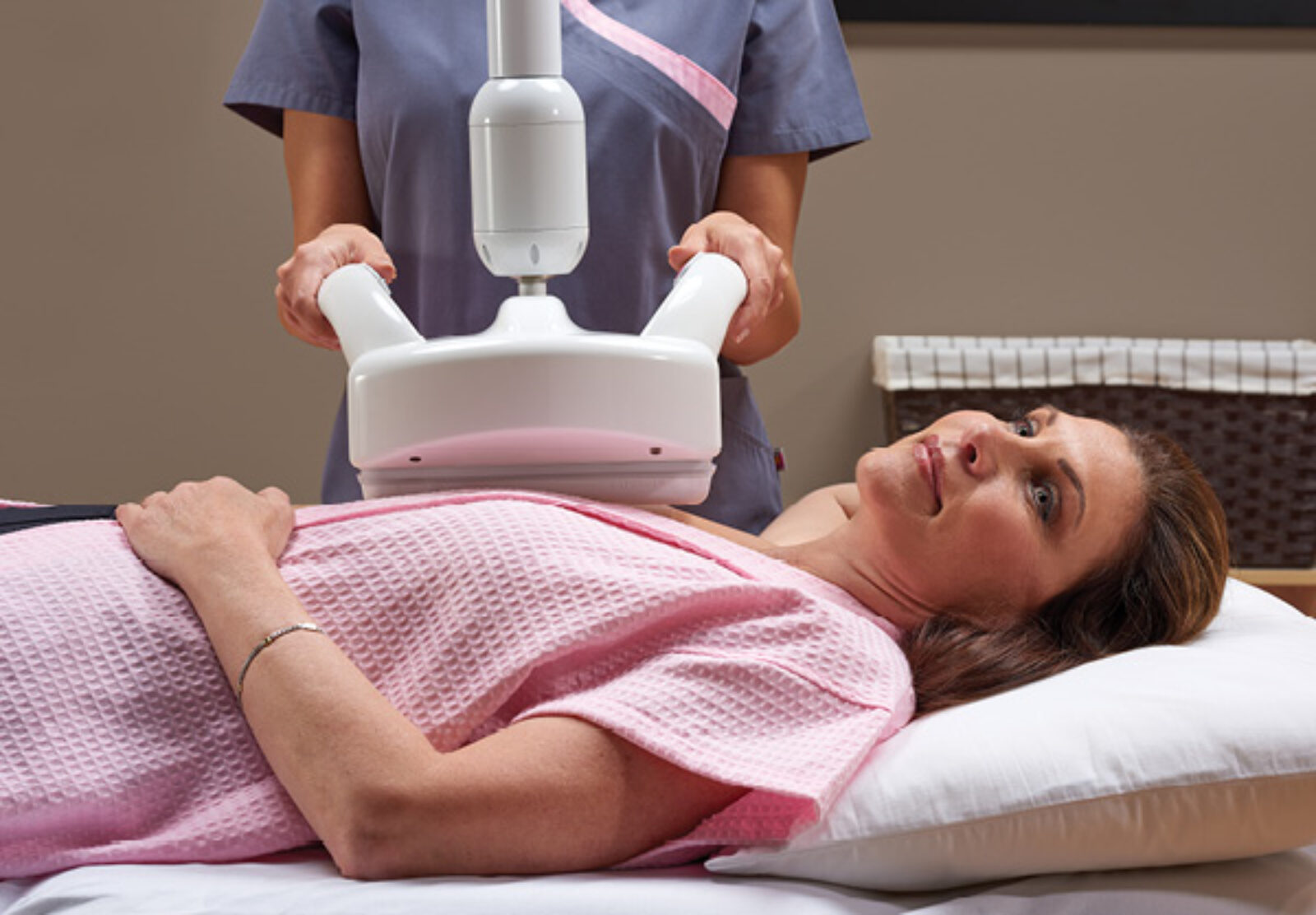
Riverside Healthcare is on the forefront of breast care by now offering a state-of-the-art breast cancer screening option (given in addition to a mammogram) for women with dense breast tissue.
“We are excited to add the Automated Breast Ultrasound system (ABUS) to our comprehensive breast imaging program,” said Ravi Ramakrishna, DO, Lead Interpreting Physician for the Riverside Breast Center. “By offering ABUS in addition to 3D mammography for our patients with dense breast tissue, we anticipate improving early detection for small cancers that may not be seen on a mammogram alone in these women.”
Dense breast tissue has been found to increase the risk for the development of cancer and also makes cancer more difficult to detect using mammography, studies show. As breast density goes up, the sensitivity of mammograms goes down. On mammograms dense tissue can result in a masking affect, making cancers more difficult to visualize. With ABUS, suspicious findings appear dark against the surrounding bright dense tissue.
In early 2019, a national density inform law was passed that mandates that the FDA update mammography reporting so that women be notified if their breasts are dense. Providers may offer supplemental imaging as appropriate to help find cancers hiding in dense breast tissue.
“Screening mammogram still remains the gold standard for the early detection of breast cancer; however dense breast tissue can result in limitations to the sensitivity of mammograms making cancers more difficult to detect. ABUS will become an important tool to improve detection.” added Dr. Ramakrishna. Designed and built specifically for screening, research shows that ABUS technology as a supplement to mammography has the potential to find additional cancers that would not have been found with mammography alone.
It is recommended that women get regular mammograms as suggested by their doctor, and if they have been informed that they have dense breast tissue, they should talk to their doctor or radiologist about their specific risk and additional screening tests that might be appropriate. The Invenia ABUS 2.0 system from GE Healthcare is FDA-approved for breast cancer screening in the United States as an adjunct to mammography for women with dense breast tissue. The system is designed to enhance the consistency, reproducibility, and sensitivity of breast ultrasound, demonstrating a 35.7 percent improvement in cancer detection (sensitivity) in women with dense breasts without prior breast intervention. (FDA PMA Approval P110006, Sept. 18, 2012.)